Answer:
A solution with a pH of 5 has
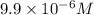 more protons in it than a solution with a pH of 7.
more protons in it than a solution with a pH of 7.
Step-by-step explanation:
The pH of the solution is negative logarithm of hydrogen ion concatenation in the aqueous solution. Mathematically written as:
![pH=-\log [H^+]](https://img.qammunity.org/2018/formulas/chemistry/high-school/y1nlg9qxar6fauop1r05a1g4xt6dhnvirc.png)
1) A solution with a pH of 5 :
![5=-\log[H^+]](https://img.qammunity.org/2018/formulas/chemistry/high-school/hlu7jtdvh12myzc01yo3ntqq5q0nd5bf1d.png)
![[H^+]=10^(-5) M](https://img.qammunity.org/2018/formulas/chemistry/high-school/pjhxfkoxzdu9m9qj7ubx8i6a1yx61x9xa5.png)
2) A solution with a pH of 7 :
![7=-\log[H^+]'](https://img.qammunity.org/2018/formulas/chemistry/high-school/jsz5gdgv5i72o17yduk3zvxfbxxzzgta24.png)
![[H^+]'=10^(-7) M](https://img.qammunity.org/2018/formulas/chemistry/high-school/urwnafa0wv281ue9k1r5o22plzau72019v.png)
![[H^+]>[H^+]'](https://img.qammunity.org/2018/formulas/chemistry/high-school/r0ebazdo5lhiu977bxwxxma1aoowb6faex.png)
Difference in concentration of hydrogen ions or protons in both solutions:
![[H^+]-[H^+]'=10^(-5)M-10^(-7) M=9.9* 10^(-6) M](https://img.qammunity.org/2018/formulas/chemistry/high-school/nxf22cedzj3fot2a900ty400620j66si1p.png)