Answer:
32 grams
Explanations:
What are diatomic elements?
Diatomic elements are elements that contain two atoms chemically bonded together.
When looking for the molar mass of a diatomic element, the number of atoms in the element is multiplied by the molar mass of the element.
For instance, oxygen is a diatomic element with the chemical symbol of O₂. This shows that the molar mass of oxygen will be given as:
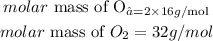
This shows that one mole of oxygen molecules would weigh 32 grams and not 16 grams.