Answer:Pressure of the air inside the sealed container will get change 2 atm when absolute temperature is doubled.
Step-by-step explanation:
According to question , Volume is constant
According to Gay Lussac's Law:
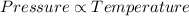 (at constant temperature)
(at constant temperature)
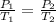
At STP, the temperature ,
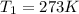
At STP, the pressure =
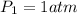
On doubling the absolute temperature the pressure changes
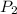 :
:
On doubling the temperature=
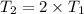
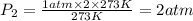
Pressure of the air inside the sealed container will get change 2 atm when absolute temperature is doubled.