Answer:
2,585.47 mL of the drink would be required to reach an LD-50 for 171 lb person.
Step-by-step explanation:
LD-50 of caffeine = 170 mg/kg body mass
Mass of the person = 171 lb = 77.56 kg
1 lb = 0.453592 kg
Lethal dose for person with mass 171 lb = 77.56 kg × 170 mg/kg =13,185.92 mg
13,185.92 mg = 13.18592 g
(1 mg = 0.001 g)
Percentage of caffeine in drink = 0.51 %
Mass of drink = M
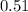
M = 2,585.47 g
Volume of the drink = V
Density of the drink = D = 1.00 g/mL
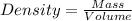
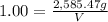
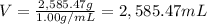
2,585.47 mL of the drink would be required to reach an LD-50 for 171 lb person.