Any buffer exists in this equilibrium
HA <=>
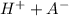
In a buffer, there is a large reservoir of both the undissociated acid (HA) and its conjugate base (
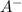
)
When a strong acid is added, it reacts with the large reservoir of the conjugate base (
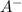
) forming a salt and water. Since this large reservoir of the conjugate base is used, the ph does not alter drastically, but instead resist the pH change.