Answer: 1) A. More H2CO3 is produced.
2) C.
![K=([SO_2]^2*[O_2])/([SO_3]^2)](https://img.qammunity.org/2018/formulas/chemistry/high-school/11lkq0cz617z9hzjehl8wdzqhvl0swc0c6.png)
Explanation:1) Any change in the equilibrium is studied on the basis of Le-Chatelier's principle.
This principle states that if there is any change in the variables of the reaction, the equilibrium will shift in the direction to minimize the effect.
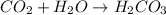
If concentration of
 is increased, that is the reactant is increased, so according to the Le-Chatlier's principle, the equilibrium will shift in the direction where decrease of concentration of
is increased, that is the reactant is increased, so according to the Le-Chatlier's principle, the equilibrium will shift in the direction where decrease of concentration of
 takes place. Therefore, the equilibrium will shift in the right direction, i.e.
takes place. Therefore, the equilibrium will shift in the right direction, i.e.
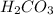 will be formed.
will be formed.
2) The rate of a equilibrium reaction is determined by equilibrium constant. Equilibrium constant is the ratio of the product of the concentration of products to the product of the concentration of reactants each term raised to their stochiometric coefficients.
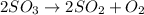
![K=([SO_2]^2* [O_2])/([SO_3]^2)](https://img.qammunity.org/2018/formulas/chemistry/high-school/d7z7kr4l5eew6h8bc6ivpiwohk9tqg5q8u.png)