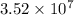Answer : The number of gold atoms will be,
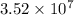
Explanation :
First we have to determine the diameter of an atom of gold.
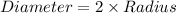
Given :
Radius of an atom of gold =
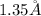
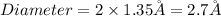
Conversion used :
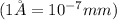
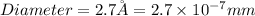
Now we have to calculate the number of gold atoms.
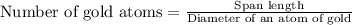
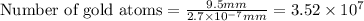
Therefore, the number of gold atoms will be