Answer: Option (d) is the correct answer.
Step-by-step explanation:
When a neutral atom gain electrons then it acquires a negative charge whereas when an atom loses electrons then it acquires a positive charge.
For example, atomic number of calcium is 20 and in order to attain stability it loses two electrons and thus its forms
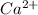 ion.
ion.
Thus, there will be decrease in the size of its radius due to loss of electrons.
Therefore, we can conclude that when electrons are removed from the outermost shell of a calcium atom, the atom becomes a cation that has a smaller radius than the atom.