Answer:
D. 4 Valence Electrons and 4 Energy shells
Step-by-step explanation:
Hello!
In this case, since finding the number of valence electrons and energy shells require the determination of the electron configuration for the element, knowing that germanium has 32 electrons (atomic number), we have:
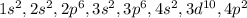
We can see that the outermost energy shell is 4 which storages 4 electrons (2 from 4s and 2 from 4p); therefore, we infer it has 4 valence electrons and 5 energy shells, so the answer is:
D. 4 Valence Electrons and 4 Energy shells.
Best regards!