Step-by-step explanation:
According to the mole concept, there are
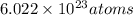 present in 1 mole of a substance.
present in 1 mole of a substance.
Mathematically, 1 mole =
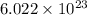 atoms or molecules
atoms or molecules
Therefore, calculate the number of moles of
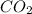 present in
present in
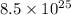 molecules as follows.
molecules as follows.
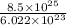
=
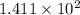 mol
mol
or, = 141.1 mol
Thus, we can conclude that there are 141.1 moles present in
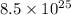 molecules of
molecules of
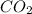 .
.