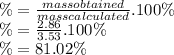Answer:
81.02%
Step-by-step explanation:
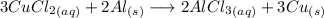
We know that 3.53 grams of Cu should be produced, but we get 2.86 grams of this compound.
The yield of a reaction is the amount of product obtained in a chemical reaction
To know the performance of a reaction in percentage we use the following formula