Answer:
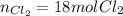
Step-by-step explanation:
Hello,
In this case, the undergoing chemical reaction is:
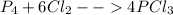
In such a way, if 3 moles of P₄ are reacting, the needed moles of Cl₂ to completely consume them turn out:
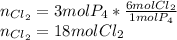
This is done by realizing that 1 mole of P₄ needs 6 moles of Cl₂ to take the reaction to completion.
Best regards.