Answer: The element having this electronic configuration is Iodine.
Step-by-step explanation:
We are given an electronic configuration of element, which means that the total number of electrons are provided.
To know the element, we need to find the atomic number of that element.
Atomic number is defined as the number of electrons or protons present in a atom.
Atomic number = Number of electrons = Number of protons
Electronic configuration :
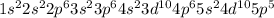
Total number of electrons = 53
The element having atomic number 53 is Iodine.
Hence, the element having this electronic configuration is Iodine.