Answer : The correct option is, (C) (iii) only
Explanation :
Entropy : Entropy measures the degree of randomness or disorderedness.
When we are going from solid to liquid state, the degree of randomness of molecule will increases. Thus, the entropy will also increases.
Similarly, when we are going from liquid to gaseous state, the degree of randomness of molecule will increases. Thus, the entropy will also increases.
But As we are going from gas to liquid to solid state, the degree of randomness of molecule will decreases. Thus, the entropy will also decreases.
Or we can say that the more that number of moles of the product, the more will be the entropy.
(i)
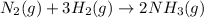
In this reaction, entropy decreases because the number of moles present on product side is lesser than the number of moles present on reactant side.
(ii)
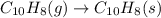
In this reaction, entropy decreases because as we are going from gas to solid state, the degree of randomness of molecule decreases.
(iii)
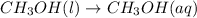
In this reaction, entropy increases because methanol liquid dissociated into ions that means the number of moles present on product side are more than the number of moles present on reactant side.
Hence, the correct option is, (C) (iii) only