Answer: Option (b) is the correct answer.
Step-by-step explanation:
A substance that dissociates into ions upon dissolving in water is able to conduct electricity due to the movement of ions or electrons.
This is because conductivity is the ability of a substance through which electricity can flow due to the free movement of electrons.
For example,
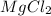 is an ionic compound and when it is dissolved in water then it readily dissociates into magnesium and chlorine ions.
is an ionic compound and when it is dissolved in water then it readily dissociates into magnesium and chlorine ions.
Therefore, it is able to conduct electricity.
Whereas
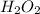 ,
,
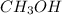 , and
, and
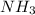 are all non-polar in nature. Hence, they do not dissociate into ions.
are all non-polar in nature. Hence, they do not dissociate into ions.
So, they do not conduct electricity.
Thus, we can conclude that out of the given options
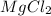 best conducts electricity in a aqueous solution.
best conducts electricity in a aqueous solution.