Step-by-step explanation:
Elements of group 18 are known as noble gases. The elements of this group are He, Ne, Ar, Kr, Xe, and Rn.
The electronic configuration of He is
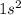 .
.
The electronic configuration of Ne is
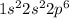 .
.
The electronic configuration of Ar is
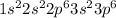 .
.
The electronic configuration of Kr is
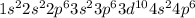 .
.
The electronic configuration of Xe is
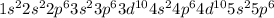
The electronic configuration of Rn is
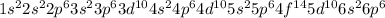 .
.
Thus, we can conclude that elements of group 18 have complete valence electron shells.