Answer : The number of atoms of argon are

Explanation : Given,
Moles of argon = 26.8 moles
As we know that,
1 mole of substance always contains
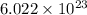 number of atoms.
number of atoms.
As, 1 mole of argon contains
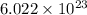 number of atoms
number of atoms
So, 26.8 mole of argon contains
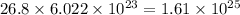 number of atoms.
number of atoms.
Therefore, the number of atoms of argon are
