Answer : Option C) The solution of salt water will have a higher conductivity than the solution of sugar water.
Explanation :
When salt (NaCl) is dissolved in water it forms cations of
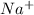 and
and
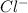 which are electrolytic ions. This occurs because the NaCl is a strong electrolyte in nature as it can get easily dissociated into its ions. Also, solutions of strong electrolytes are observed to be good conductors of electricity as they contain a comparatively high concentration of ions.
which are electrolytic ions. This occurs because the NaCl is a strong electrolyte in nature as it can get easily dissociated into its ions. Also, solutions of strong electrolytes are observed to be good conductors of electricity as they contain a comparatively high concentration of ions.
But in case of sugar, it is a non-electrolyte, which means it does not produces ions when dissolved in water. As the solution of sugar contains molecules of sucrose, but does not gets dissociated into ions. This absence of ions in a sugar aqueous solution makes it a non electrolytic in nature.