Answer : The correct option is, (C) 1.173
Solution : Given,
Molar mass of carbon = 12.011 g/mole
Molar mass of oxygen = 15.999 g/mole
Molar mass of carbon dioxide gas,
 = 44 g/mole
= 44 g/mole
Molar mass of oxygen gas,
 = 31.998 g/mole
= 31.998 g/mole
According to the Graham's law, the rate of effusion of a gas is inversely proportional to the square root of the molar mass of a gas.
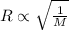
or,
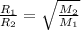
where,
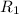 = rate of effusion of a carbon dioxide gas
= rate of effusion of a carbon dioxide gas
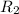 = rate of effusion of oxygen gas
= rate of effusion of oxygen gas
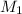 = molar mass of a carbon dioxide gas
= molar mass of a carbon dioxide gas
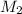 = molar mass of oxygen gas
= molar mass of oxygen gas
Now put all the given values in the above formula, we get the molar mass of a gas.
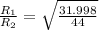
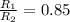
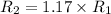
Therefore, the 1.17 times greater is the rate of effusion of oxygen gas than that of carbon dioxide gas at the same temperature and pressure.