Answer:
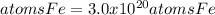
Step-by-step explanation:
Hello,
In this case, considering that for iron the grams are related with moles via the atomic mass and the atoms with moles via the Avogadro's number, we develop the following proportional analysis to compute the required atoms:
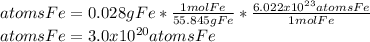
Best regards.