Answer:
0.012036 moles of nitric acid will be produced when 0.65 grams of dinitrogen pentoxide reacts.
Step-by-step explanation:
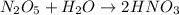
Moles of dinitrogen pentoxide =
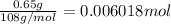
According to recation , 1 mole of dinitrogen pentoxide gives 2 moles of nitric acid.
Then 0.006018 moles of dinitrogen pentoxide will give:
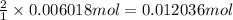 of nitric acid
of nitric acid
0.012036 moles of nitric acid will be produced when 0.65 grams of dinitrogen pentoxide reacts.