Answer:
136.15 g/mol is the molar mass of calcium sulfate.
Step-by-step explanation:
Molar mass is defined as a total sum of atomic masses of all and each type of atoms constituting the molecule.
Atomic mass of calcium = 40.08 g/mol
Atomic mass of sulfur = 32.07 g/mol
Atomic mass of oxygen = 16.00 g/mol
Molar mass of calcium sulfate, (
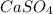 ) :
) :
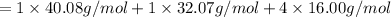
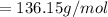
136.15 g/mol is the molar mass of calcium sulfate.