Answer:
The expected freezing point of a 0.50 m solution of sodium sulfate is -0.93°C.
Step-by-step explanation:
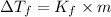
where,
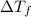 =depression in freezing point =
=depression in freezing point =
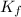 = freezing point constant
= freezing point constant
m = molality
we have :
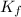 =1.86°C/m ,
=1.86°C/m ,
m = 0.50 m
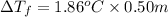
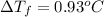
Freezing point of pure water = T = 0°C
Freezing point of solution =
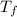
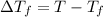
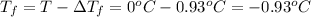
The expected freezing point of a 0.50 m solution of sodium sulfate is -0.93°C.