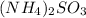Answer: The chemical formula for the compound is
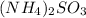
Step-by-step explanation:
An ionic compound is defined as the compound which is formed when electron gets transferred from one atom to another atom.
These are usually formed when a metal reacts with a non-metal or a metal reacts with a polyatomic ion or a reaction between two polyatomic ions takes place.
We are given:
Two polyatomic ions having formulas
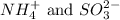
By criss-cross method, the oxidation state of the ions gets exchanged and they form the subscripts of the other ions. This results in the formation of a neutral compound.
Hence, the chemical formula for the compound is