You must see the mass of Gallium in the periodic table. The mass present there is calculated by a weighted average of the mass of the isotopes, where the weights are the abundances of each isotope. Let X the isotopic abundance of Ga⁷¹. Since there are only Ga⁶⁹ and Ga⁷¹, the isotopic abundance of Ga⁶⁹ is 1-X. Then:
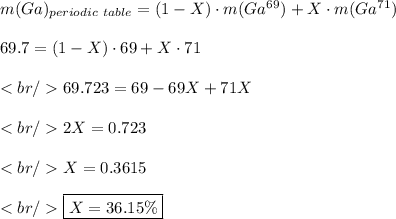
I don't know the options, but probably 40% is the closest to 36.15%.