Answer:
4.4x10¹⁹
Step-by-step explanation:
Hello,
In this case, if we consider the four significant figures Avogadro's number has, the maximum number that can be added without changing the displayed value is 4.4x10¹⁹ as the result will be:
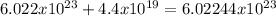
Even when the value changes, the added fours do not round the last two of the Avogadro's number, thus, it will remain technically the same.
Best regards.