Answer : The difference is that the selenium has more number of electrons as compared to aluminum.
Explanation :
The difference between the number of electrons in an atom of selenium, Se, and the number of electrons in an atom of aluminum, Al are:
As we know that,
Element selenium has the atomic number 34 while the aluminium has atomic number of 13.
Element selenium belongs to the group 16 while aluminium belongs to group 3. The group determine the number of electron in its outermost shell.
This means that, selenium has six electrons in its outermost shell and aluminium has only three electrons in its outermost shell.
The electronic configuration of selenium is,
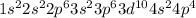 while electronic configuration of aluminium is,
while electronic configuration of aluminium is,
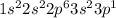
Thus, the difference between the number of electrons in an atom of selenium and the number of electrons in an atom of aluminum is that the selenium has more number of electrons as compared to aluminum.