Answer: The mass of 1 mole of
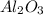 is 101.96 amu.
is 101.96 amu.
Step-by-step explanation:
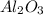 is a compound. One mole of a compound has a mass which is equal to the molecular weight (molar mass) of the compound expressed in 'amu' and it contains
is a compound. One mole of a compound has a mass which is equal to the molecular weight (molar mass) of the compound expressed in 'amu' and it contains
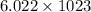 molecules of that compound.
molecules of that compound.
To calculate the molar mass of the compound, we multiply the number of atoms of an element with its atomic mass. Molar mass of
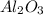 will be:
will be:
Atomic mass of Aluminium = 26.98 amu
Number of atoms of Aluminium = 2
Atomic mass of Oxygen = 16.00 amu
Number of atoms of Oxygen = 3
Molecular weight of
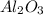 =
=
![[(2* 26.98)+(3* 16.00)]](https://img.qammunity.org/2018/formulas/chemistry/high-school/87sbydoms3au7l352dcinqzb0hvbbix4qe.png) = 101.96 amu
= 101.96 amu
Hence, the mass of 1 mole of
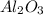 is 101.96 amu.
is 101.96 amu.