Answer:
15 grams
Step-by-step explanation:
Mass of Cu = 10 g
Molar mass of Cu = 63.546 g/mol
Moles of Cu =
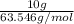 = 0.1574
= 0.1574
Mass of I₂ = 10 g
Molar mass of I₂ = 253.8 g/mol
Moles of I₂ =
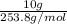 = 0.04
= 0.04
I₂ here is the limiting reactant as it has the least number of moles.
Based on the balanced equation,
1 mole of I₂ = 2 moles of CuI
0.04 mole of I₂ = 0.08 moles of CuI
Molar mass of CuI = 190.45 g/mol
Mass of CuI = 0.08 moles x 190.45 g/mol = 15.236 g