Answer:
Volume of H2 produced = 26.5 L
Step-by-step explanation:
Step 1: Calculate the moles of H2 produced
The given reaction is:
2Li + 2H2O → 2LiOH + H2
Here Li is the limiting reagent since H2O is in excess
Based on the reaction stoichiometry:
2 moles of Li produces 1 mole of water
Mass of Li = 21.3 g
Atomic mass of Li = 7 g/mol
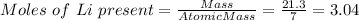
Therefore, moles of H2O produced will be:
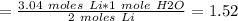
Step 2: Calculate the volume of H2 produced
Based on the ideal gas equation:
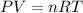
where P = pressure = 1.40 atm
T = Temperature = 297 K
n = number of moles = 1.52
R = gas constant = 0.0821 Latm/mol-K
