Answer :
First we have to determine the hybridization of the following molecules.
Formula used :
![\text{Number of electron pair}=(1)/(2)[V+N-C+A]](https://img.qammunity.org/2018/formulas/chemistry/college/zia3jm1s1xbt3kxm0r43raz35v736wepqh.png)
where,
V = number of valence electrons present in central atom
N = number of monovalent atoms bonded to central atom
C = charge of cation
A = charge of anion
(1) The given molecule is,
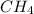
![\text{Number of electrons}=(1)/(2)* [4+4]=4](https://img.qammunity.org/2018/formulas/chemistry/college/cxsedm4go9bd1gwy18as0n6kvc6x3pdiov.png)
The number of electron pair are 4 that means the hybridization will be
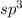 and the electronic and molecular geometry of the molecule will be tetrahedral. The bond angle is
and the electronic and molecular geometry of the molecule will be tetrahedral. The bond angle is
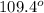
(2) The given molecule is,

![\text{Number of electrons}=(1)/(2)* [6+2]=4](https://img.qammunity.org/2018/formulas/chemistry/college/d21c1pm6xm9eiz8o6rqw2fyjkujgzrup8h.png)
The number of electron pair are 4 that means the hybridization will be
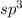 and the electronic geometry of the molecule will be tetrahedral.
and the electronic geometry of the molecule will be tetrahedral.
But as there are 2 atoms around the central oxygen atom, the third and fourth position will be occupied by lone pair of electrons. The repulsion between lone and bond pair of electrons is more and hence the molecular geometry will be bent or angular.
From this we conclude that these molecules have approximately the same bond angles but different molecular shape due to repulsion between lone and bond pair of electrons is more in
 . Thus, the bond angle which was supposed to be
. Thus, the bond angle which was supposed to be
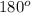 but it decreases to
but it decreases to
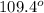 .
.