Answer:
One could you seperate the sodium from the chlorine by electrolysis.
Step-by-step explanation:
Molten salt electrolysis works by letting the ions flow trhough a liquid medium, thus possibilitating electrolysis. It is required to do this way because in aquous solution Hydrogen is produced istead of Sodium and in solid phase there is not enough ion mobility to possibilitate electrolysis.
Sodium ions goes to the cathode, where it is reduced to sodium metal
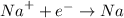
Chloride ions migrate to the anode, where they give up their electrons and are oxidized to chlorine gas:
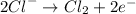
The overall reaction is the decomposition of sodium chloride into its elements:
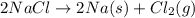
A diagram is attached, to enhance comprehention.