Answer : Option B) as a Brønsted-Lowry base by accepting a proton.
Explanation : In the given reaction
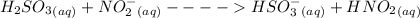
According to the Bronsted-Lowry theory of acids and bases, the formation of conjugate acids and bases forms the central theory.
Wherein, the conjugate base is the ion or molecule which remains after the acid has lost its proton, and the conjugate acid is the species which is created when a base accepts that proton.
In the given question
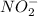 is the species which is acting as a Bronsted-Lowry base, as this the species which is accepting a proton and getting converted into
is the species which is acting as a Bronsted-Lowry base, as this the species which is accepting a proton and getting converted into
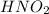 .
.