Answer: "
 " atoms of aluminum (Al) .
" atoms of aluminum (Al) .
____________________________________
Step-by-step explanation:
_________________________
Using a technique called "dimensional analysis" ; as follows:
________________________
→ "
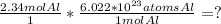
________________________
The units "mol Al" ["moles of aluminum"] cancel out; and we have:
________________________
→ "
![[(2.34) * (6.022 * 10^(23))] = ?](https://img.qammunity.org/2022/formulas/chemistry/high-school/d5lp6ifhu2xduz7qx79i5hogm8taxx96tz.png) atoms of Al [aluminum].
atoms of Al [aluminum].
________________________
Note that by definition: 1 (one) "mole" (abbreviated "mol") of anything consists of "
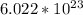 " units " of that particular thing.
" units " of that particular thing.
This number: 6.022*10^{23} —is known as: "Avogadro's number".
________________________
→ [ 2.34 * 6.022 * 10^23] ;
= 1.409148 * 10^24 ;
→ Round to 3 (Three) significant figures;
→ since: "2.34" has 3 (Three) significant figures;
→ to get: "
 atoms of aluminum (Al).
atoms of aluminum (Al).
________________________
Hope this is helpful to you! Wishing you the best!
________________________