Solving with Moles
M = mol/L
To solve for the number of atoms in a certain amount of moles, we must multiply the number of moles by Avogadro's number (
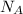 ):
):
 , and multiply the product by the number of atoms in the compound.
, and multiply the product by the number of atoms in the compound.
Solving the Question
We're given:
- Sucrose =
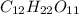
- 800.0 mL
- 0.10 M
First, we must find the number of moles. We can do this by first converting 800.0 mL to L and multiplying it by 0.10 M.
800.0 mL ÷ 1000 = 0.8 L
⇒ Now, multiply 0.8 L by 0.10 M:
0.8 L × 0.10 M = 0.08 mol
Now, we must find the number of molecules in this sample. Multiply 0.08 mol by Avogadro's number:

Finally, to find the number of atoms in the sample, multiply the number of molecules by the number of atoms in the compound.
We can begin by finding the number of atoms in sucrose:
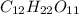
12 + 22 + 11 = 45 atoms
⇒ Multiply 45 atoms by
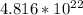 mol:
mol:
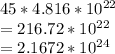
Convert to the correct number of significant figures (2):
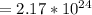
Answer
 atoms
atoms