Answer: 58 grams of
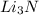 will react to produce 120 grams of LiOH.
will react to produce 120 grams of LiOH.
Explanation: Moles can be calculated by using the formula:
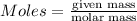 ...(1)
...(1)
Molar mass of LiOh = 23.95 g/mol
Given mass of LiOH = 120g
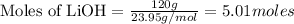
For a given chemical reaction:
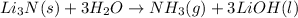
By Stoichiometry,
3 moles of LiOH is produced by 1 mole of
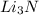
5.01 moles of LiOH will be produced by
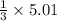 =1.67 moles of
=1.67 moles of
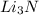
Mass of
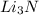 will be calculated using the equation 1:
will be calculated using the equation 1:
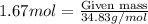
Mass of
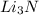 = 58.166 grams.
= 58.166 grams.