Answer: Option (a) is correct.
Step-by-step explanation:
Molecules of sugar given =
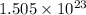
According to Avogadro's number it is known that 1 mole contains
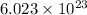 .
.
Therefore, calculate the moles of sugar present in the sample as follows.
Number of moles =
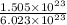
= 0.25 mol
Thus, the moles of sugar present in the sample is 0.25 mol.