Answer:
Na+ + e- Na = -2.71 V
Step-by-step explanation:
We have to remember the nomenclature for redox reactions:
Oxidation:
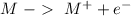
Reduction:
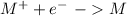
So, all the answer options are written as reductions. Therefore the voltage values, in this case, would be the reduction potentials. If we have a higher (positive) potential the compound would be more inclined to a reduction and vice-versa if we have a smaller potential (negative) the compound would be more inclined to oxidation.
In this case, the most negative value is for Na+, so this atom would be more easily oxidized.