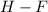Answer: The most polar bond is
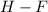
Step-by-step explanation:
Polarity of a bond is judged on the basis of electronegativity difference between the elements forming a bond.
More the electronegativity difference, more polar will be the bond and vice-versa.
The electronegativities of the following elements are:
Electronegativity of Hydrogen = 2.1
Electronegativity of Fluorine = 3.98
Electronegativity of Chlorine = 3.16
Electronegativity of Bromine = 2.96
Electronegativity of Iodine = 2.66
Electronegativity difference = 3.98 - 2.1 = 1.88
Electronegativity difference = 3.16 - 2.1 = 1.06
Electronegativity difference = 2.96 - 2.1 = 0.86
Electronegativity difference = 2.66 - 2.1 = 0.56
From the above calculation, it is visible that the electronegativity difference of hydrogen fluoride is the maximum, so the most polar bond will be of H-F only.
Hence, the most polar bond is