Answer: Option (a) is the correct answer.
Step-by-step explanation:
General electronic configuration of group 2 elements is
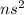 . And valence electrons are the electrons present in outermost shell of an atom.
. And valence electrons are the electrons present in outermost shell of an atom.
For example, magnesium is a group 2 elements and has electronic configuration
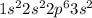 .
.
Thus, we can see that there are 2 electrons in the outermost shell of group 2 elements.
Hence, we can conclude that the elements in group 2 all have 2 valence electrons.