Answer:
the temperature of gas inside the container is 195.2 K.
Step-by-step explanation:
Given;
volume of gas in the container, V = 3.45 L
pressure of gas in the container, P = 2.14 atm
number of gas moles, n = 0.461 mol
The temperature of gas inside the container is calculated as;
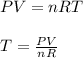
where;
R is gas constant = 0.08205 L.atm/mol.K
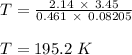
Therefore, the temperature of gas inside the container is 195.2 K.