Answer:
32.5 grams will be left after 100 minutes.
Explanation:
Given : The half–life of rubidium–89 is 15 minutes. If the initial mass of the isotope is 250 grams.
To find : How many grams will be left after 100 minutes?
Solution :
Let the exponential equation of rubidium is
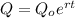
Where,
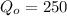 is the initial value
is the initial value
t is the time taken i.e. t=15 minutes
The half–life of rubidium–89 is 15 minutes.
i.e.
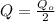
Substitute in the formula,
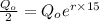
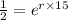
Taking log both side,
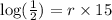
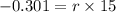
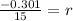
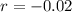
Now, we have to find Q in 100 minutes,
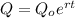
Substitute in the formula,
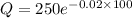
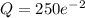
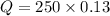
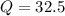
Therefore, 32.5 grams will be left after 100 minutes.