Answer: There will be no chemical reactivity between potassium metal and helium gas.
Step-by-step explanation:
Reactivity is defined as the tendency of an atom to release or gain electrons.
Potassium is a metal and its reactivity is defined as the tendency to loose electrons. Electronic configuration of potassium is:
![[Ar]4s^1](https://img.qammunity.org/2018/formulas/chemistry/high-school/emdyj31bb0bv9m2t25uxgi11dg9n2j8l1v.png) . This element will easily loose 1 electron to attain stable electronic configuration. Hence, it is more reactive.
. This element will easily loose 1 electron to attain stable electronic configuration. Hence, it is more reactive.
Helium is a noble gas and noble gases are not reactive because of the stable electronic configuration. The electronic configuration of helium is:
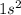 . This element is not reactive at all.
. This element is not reactive at all.
Hence, helium will not react with potassium and therefore, there will be no chemical reactivity between potassium metal and helium gas.