Answer:
Step-by-step explanation:
Hello!
In this case, since the percent composition analysis provide the by-mass percent of each atom in the molecule, we can assume we have 52 g of carbon, 13 g of hydrogen and 35 g of oxygen, so we are able to compute the moles based on their atomic masses:
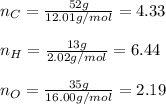
Now we divide all the moles by those of oxygen as the fewest ones in order to obtain their subscripts in the empirical formula:
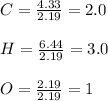
Thus, the empirical formula is:
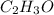
Best regards!