Answer: HBr is a powerful and dangerous acid
Step-by-step explanation:
HBr gives hydronium ions (
 ) in its aqueous solution and presence of large amount of ions in the solution makes it corrosive in nature. This is because when these ions present in the solution comes in contact with any of the objects they tends to neutralize their charge and due to this action of ions we observe corrosive nature of an acid.
) in its aqueous solution and presence of large amount of ions in the solution makes it corrosive in nature. This is because when these ions present in the solution comes in contact with any of the objects they tends to neutralize their charge and due to this action of ions we observe corrosive nature of an acid.
Chemical equation for HBr dissociating into ions is given as:
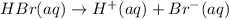
Hence ,HBr is a powerful and dangerous acid. Where as
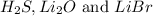 are gas , basic oxide and salt respectively.
are gas , basic oxide and salt respectively.