Answer:
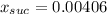
Step-by-step explanation:
Hello!
In this case, since the mole fraction relates the moles of solute and the moles of solution, and for sucrose it is:
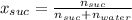
Whereas we need to compute the moles of both sucrose and water based on their molar masses (342.3 and 18.02 respectively) as shown below:
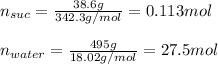
Thus, the mole fraction sucrose turns out:
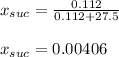
Best regards!