Answer : The density of metal will be 3.963 g/mL
Explanation : Given,
Mass of metal = 17.676 g
Initial volume of chloroform = 11.00 mL
Final volume of chloroform = 15.46 mL
First we have to calculate the volume of metal.
Volume of metal = Final volume of chloroform - Initial volume of chloroform
Volume of metal = 15.46 - 11.00 = 4.46 mL
Now we have to calculate the density of metal.
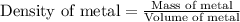
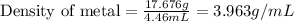
Therefore, the density of metal will be 3.963 g/mL