Answer:
The numder of moles of 7.89*10¹¹ atoms of Carbon is 1.31*10⁻¹².
Step-by-step explanation:
Avogadro's Number or Avogadro's Constant is called the number of particles that make up a substance (usually atoms or molecules) and that can be found in the amount of one mole of said substance. Its value is 6.023*10²³ particles per mole. Avogadro's number represents a quantity without an associated physical dimension, so it is considered a pure number that allows describing a physical characteristic without an explicit dimension or unit of expression. Avogadro's number applies to any substance.
Then you can apply the following rule of three, if by definition of Avogadro's number 6.023*10²³ atoms are present in 1 mole of Carbon, 7.89*10¹¹ atoms will be present in how many moles?
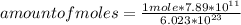
amount of moles=1.31*10⁻¹²
The numder of moles of 7.89*10¹¹ atoms of Carbon is 1.31*10⁻¹².