Answer:
The required mass of oxygen to react with 4 moles of Mg is 64 grams
Step-by-step explanation:
You know the reaction where magnesium is burned with oxygen to form magnesium oxide:
2Mg +
 --> 2MgO
--> 2MgO
Oxygen is required to react completely (that is, sufficient oxygen must be present for the reaction to occur) with 4 moles of Mg. For that you observe the stoichiometry of the reaction, the amount of reagents needed. Stoichiometry in this case tells you that in order for 2 moles of Mg to react, you need 1 mole of
 . Taking this into account, you apply a rule of three: if to react with two moles of Mg you need 1 mole of
. Taking this into account, you apply a rule of three: if to react with two moles of Mg you need 1 mole of
 , how many moles of
, how many moles of
 do you need to react with 4 moles of Mg?
do you need to react with 4 moles of Mg?
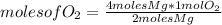
moles of O_{2} =2 moles
To know the mass of
 that reacts, you must take into account the molar mass of oxygen, which is 32 grams / mol and you can apply another rule of three: if in a mole of
that reacts, you must take into account the molar mass of oxygen, which is 32 grams / mol and you can apply another rule of three: if in a mole of
 there are 32 grams, how many grams are in 2 moles of oxygen?
there are 32 grams, how many grams are in 2 moles of oxygen?
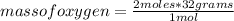
mass of oxygen= 64 grams
Then, the required mass of oxygen to react with 4 moles of Mg is 64 grams