Answer: 4.544 grams of chlorine gas is produced in the given reaction.
Explanation: Number of moles can be calculated by using the formula:
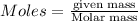 ... (1)
... (1)
Molar mass of NaCl = 58.5 g/mol
Given mass of NaCl = 7.5 grams
Moles of NaCl is calculated by:
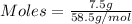
Moles of NaCl = 0.128 moles
The given equation is not balanced. For any further calculation, we first need to balance the equation.
The balanced chemical equation follows:
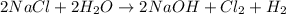
By Stoichiometry,
2 moles of NaCl produce 1 mole of chlorine gas
So, 0.128 moles of NaCl will produce =
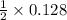 = 0.064 moles of chlorine gas
= 0.064 moles of chlorine gas
Mass of Chlorine gas is calculated by using equation 1, we get
Molar mass of
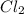 = 71 g/mol
= 71 g/mol
Putting the values in above equation, we get
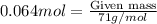
Mass of
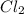 produced = 4.544 grams
produced = 4.544 grams